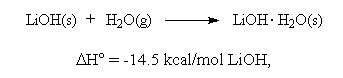
Lithium Hydroxide (LiOH) is the most commonly used CO2 sorbent for use in expendible devices. Two types of LiOH cannisters (one square and one round) played an important role during the Apollo 13 Mission (as dramatized in the movie). Expendible LiOH cannisters were only recently replaced aboard the Shuttle Orbiter by a Vacuum Regenerable Solid Amine based system. LiOH is currently used in the EMU (Extravehicular Mobility Unit) life support backpack worn by astronauts during EVA.
The presence of water vapor is important to the functioning of LiOH beds. Chemisorption of CO2 is thought to take place via a two step reaction in which lithium hydroxide monohydrate is first formed by the exothermic reaction,
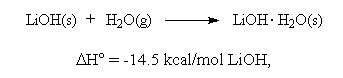
followed by the endothermic formation of lithium carbonate.
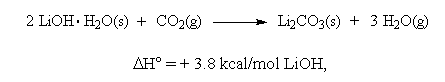
Significantly, water is required on both sides of these reaction equations. For the net reaction,
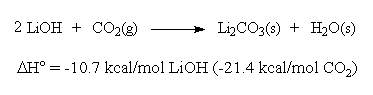
two moles of water are liberated for each mole of CO2 chemisorbed.
Author: Tugrul Sezen BACK TO COURSE MAIN PAGE
[email protected]
BACK
TO SPACE SETTLEMENT HOME PAGE
|
Curator: Al Globus If you find any errors on this page contact Al Globus. |
 |
This site was hosted by the NASA Ames Research Center from 1994-2018 and is now hosted by:
